You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The control of pain, its diagnosis, and treatment of its causes is an important obligation for dental professionals. Yet many patients who report that they have chronic orofacial pain can be easily dismissed, misdiagnosed, and/or treated incorrectly as the etiology for their symptoms remains shrouded in mystery. This leaves the patient frustrated, disappointed, and, worst of all, still in chronic pain.
Orofacial Pain
By definition, orofacial pain is associated with the hard and soft tissues of the head, face, and neck. When any of these tissues receive noxious stimulation, impulses are sent through the trigeminal nerve to the brain.1 Brain circuits primarily responsible for processing complex behavior interpret these signals as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”2
The density of the anatomic structures in this region of the body makes diagnosis a complex process. It is quite common for patients to describe the site where they are feeling pain and be totally unaware that the source is elsewhere.3 The referred-pain phenomenon is caused by the convergence of multiple sensory nerves that carry input to the trigeminal spinal nuclei from cutaneous and deep head and neck tissues.1,3
Toothache pain is among the most common forms of orofacial pain.4 Once toothache pain is ruled out, however, TMDs and headaches rise to the top of the list. Many times, these can all occur together in a comorbid situation. In addition, fibromyalgia, chronic fatigue syndrome, or any other condition that presents with chronic pain will further complicate the clinician’s ability to determine the causality.5 Diagnosis in these cases is difficult but is best achieved like “peeling an onion”—eliminating the symptoms one layer at a time.
The Temporomandibular Joint
The temporomandibular joint (TMJ) is a complex joint that provides both rotational and gliding movements of the mandible. Structurally, it is composed of the mandibular condyle designed to fit into the glenoid fossa of the temporal bone. An articular disc made of dense fibrocartilage separates the bones from making direct contact with each other. Blood vessels and nerves are not present in the anterior portion of the disc. However, the posterior portion of the disc has rich innervation and is quite vascular. The joint is lubricated by synovial fluid.1
The muscles of mastication are responsible for the movement of the TMJ. They are one of the major muscle groups in the head, with the other being the muscles of facial expression. The four muscles of mastication are the masseter, temporalis, medial pterygoid, and lateral pterygoid.3
Temporomandibular Disorders
Temporomandibular disorders (TMD) are a group of musculoskeletal and neuromuscular disorders that predominantly involve the functional joint, muscles, and disc of the TMJ. TMD should be considered in every differential diagnosis of facial pain because it is the most common cause of nondental pain.
Proper diagnosis requires a detailed history of onset, duration, what makes it better, and what makes it feel worse. Along with persistent jaw pain, patients will commonly report earache, headache, and diffuse facial pain. In addition, they may complain of radiating pain or stiffness in the face, jaw, or neck, limited movement or locking of the jaw, painful clicking, popping or grating in the jaw joint when opening or closing the mouth, and possibly changes in the way that their teeth fit together. These symptoms can be worse when patients awaken in the morning or can gradually worsen throughout the day.
Threshold, localization, pain sensitivity, and description of pain vary greatly from patient to patient due to both genetic and environmental factors. This fact, coupled with the complexity of the pain mechanism itself, highlights the importance of proper diagnosis and treatment of each patient’s case.6
Because one of the essential keys of problem solving in this arena is the history of the illness, the patient interview needs to be performed by the treating dentist. This gives the patient a chance to tell his or her story and will undoubtedly reveal many factors that influence the manifestation of the condition. This is also a way to casually observe the patient’s lip and jaw habits, facial expressions, and posture, and can reveal much about the patient’s emotional status, such as frustration with the pain. By validating these concerns, the dentist will build rapport and trust.
Historically, malocclusion has been considered a primary cause of TMD. However, recent studies have shown that poor occlusion accounts for a low incidence of cases.7-9
TMDs have many classification systems. In simple terms, the pain is either arthrogenous or myogenous. Arthrogenous (joint and disc) TMDs are most commonly caused by disc displacement or occur secondary to degenerative disc diseases, anklyosis, dislocation, infection, or neoplasia. The underlying cause for myogenous TMDs is muscular hyperactivity and dysfunction secondary to bruxism, hypermobility, or external stressors. Myogenous TMD can cause ischemia in the facial skeletal muscles. Irreversible muscle cell damage can begin after approximately 3 hours of ischemia and are paralleled by progressive microvascular damage in the facial skeletal muscles. This, in turn, can add to the facial pain and the degenerative pathophysiology cycle. Patients with myogenous TMD will report more comorbid disorders and more severe pain than patients with arthrogenous TMD.10 Hence, frontline TMJ therapy focuses on the treatment of the hyperactivity in the muscles of mastication.
Myofascial Pain Syndrome
According to the National Institute of Dental and Craniofacial Research, the most common form of TMD is myofascial pain syndrome (MPS).11 This chronic inflammatory disorder affects both muscle and fascia. Repetitive motions, injury to muscle fibers, and excessive strain on ligaments and tendons are the primary causes. Patients also frequently report depression or fatigue and may exhibit behavioral changes. What differentiates TMD-related MPS from other muscle pain syndromes is the presence of trigger points that have the ability to refer the pain to other areas of the head and neck.
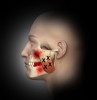
Trigger points (Figure 1 through Figure 4) are the result of excessive muscle contraction and dysfunction of the motor endplate. This type of muscle spasm in a muscle is different from the entire muscle being tight. Because of the localized overcontraction, the blood flow to the immediate area stops. This, in turn, results in a restriction of the blood supply (ischemia). The accumulation of metabolic waste products and toxins sensitizes the trigger point, causing it to send pain signals and further increase contraction. Thus, the physiology of a trigger point involves a vicious cycle of a metabolic crisis.
Clinically, trigger points can be identified by examining signs, reproducing symptoms, and performing manual palpation. Firm palpation of the muscle belly usually results in the location of one or more sore, nodular areas within a tight band of muscle fibers. A twitch response is often elicited when pressure is applied followed by the spread of referred pain.12
Masseter Muscle
The masseter is the major muscle of mastication and derives its name from the Greek word meaning “to chew.” The mandible is the only bone of the skull that is actually moveable, while the maxilla remains fixed; thus, the masseter is constantly in use. Located on each side of the face in the parotid region at the back of the jaw, these muscles are easily visible or palpable when the patient clenches the jaw, as they contract strongly just in front of the lower portion of the ears.
The average human can bite with a force of 150 pounds, and bites of more than 250 pounds are within the norm. The masseter achieves these seemingly impossible forces because it has the mechanical advantage of a lever arm that is much shorter than other muscles. Because it is highly active, the masseter is likely to tense when a person is emotionally distressed, concentrating, or angry. When the tension endures for extended periods, the development of MPS trigger points is common.
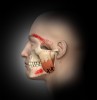
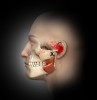
In general, masseter trigger points cause pain in the eye, face, jaw, and teeth. An unexplained earache can be a result of masseter trigger points, and research by Simons et al12 reports trigger points of the masseter can even cause an itch deep in the ear. Trigger points in the deep layer of the masseter may also be a cause of tinnitus (ringing noise in the ear with no cause). Figure 1 through Figure 3 illustrate trigger points in masseter muscle and the common referral patterns (shown in red).12 It is important to note that the facial nerve is a motor nerve that innervates the muscles of facial expression. Care should be taken to know the facial nerve anatomy when treating trigger points in the deep masseter near the zygomatic arch.
Temporalis Muscle
The temporalis is a large, thin fan-shaped muscle located in the side of the skull above and in front of the ear. Although the masseter is the more powerful muscle, the temporalis is a large and important chewing muscle. It starts at the temporal bone of the skull but passes all the way down beneath the zygomatic arch (cheek bone), attaching to the mandible, enabling it to assist the masseter in closing the jaw but also to retract the mandible. Before treating this area, the clinician should bear in mind that the temporal branch of the facial nerve mentioned above runs through the anterior temporalis.
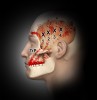
By placing your fingers just above your ear while clenching and unclenching your jaw, you will be able to feel the temporalis at work. If you clench your jaw very tightly, you will feel a powerful contraction in the temporalis. Figure 4 illustrates how significantly temporalis trigger points can refer to the upper teeth as well as the head, cheek, eye, and ear areas. Often, if this is mistaken for odontogenic pain, root canals might be performed. However, in these cases, a patient’s pain persists because of the incorrect diagnosis and treatment.
Treating With Botulinum Neurotoxin Type A
In the spirit of “do no harm,” noninvasive and reversible modalities should be used as frontline treatment.13 Many palliative treatments can be used alone or in combination with each other to manage TMD pain. These include (but are not limited to) splint therapy, massage, physical therapy, biofeedback, acupuncture, chiropractic therapy, spray and stretch with ethyl chloride, antidepressants, narcotics, and nonsteroidal anti-inflammatory drugs.14 However, the use of botulinum neurotoxin type A (BoNT-A) could also be considered. In addition to its well-publicized cosmetic uses, BoNT-A (Botox, Dysport, Xeomin) has been approved by the US Food and Drug Administration (FDA) for painful conditions potentially related to TMD, such as cervical dystonia and migraine.15,16
BoNT-A is an injectable pharmaceutical agent derived from the bacterium clostridium botulinum. Given in small doses, this purified protein can be used to selectively relax the strength of skeletal muscles by interfering with the release of acetylcholine at the neuromuscular junction. Hence, the muscle will not be able to contract with the same intensity because the amount of available neurotransmitter has been reduced. As stated above, the constant, sometimes dysfunctional, contraction of the muscles of mastication can be the primary cause of the trigger point in MPS-related TMD. When BoNT-A is placed in several spots in the belly of the muscles, it will reduce the hyperactivity in the muscle and, in turn, reduce the patient’s pain.17
Treatment with BoNT-A for TMD takes a week or so to work and will last 3 to 4 months. The treatment will then wear off without any negative consequences. Normal functions such as speaking, swallowing, and biting are left unaffected, while there will be a reduction in pain and discomfort. Unlike systemic medications that affect the patient’s entire body, this treatment can focus on the source of the problem. Both active and latent trigger points respond well to these injections, and the patient will periodically report immediate pain relief from the injection itself because it has a “dry needling” effect. While TMD has no cure, patients who receive regular treatment with BoNT-A find that the effects of the treatment become longer lasting as time goes by. This therapy has been used successfully on many patients who have not responded to other treatments.18
Practitioners considering using BoNT-A for frontline TMJ therapy and orofacial pain would benefit from taking a course with one-on-one mentored live-patient training. Such a course should include the anatomy, physiology, pharmacology, adverse reactions, and potential complications involved with these treatments. The cost of commercially available BoNT-A to a practitioner is approximately $600 for a 100-unit vial. Before using BoNT-A, it is also imperative that practitioners take responsibility for following the regulations set by the board of dentistry and laws of the state where they practice.19
Bruxism and Dental Sleep Medicine
Oral parafunction is the habitual use of any part of the mouth, tongue, and jaw that is unrelated to eating, drinking, and speaking. The most common parafunctional habit is bruxism, also known as clenching and grinding. These destructive forces have been linked with TMD for several reasons. The amount of pressure placed on teeth during functional habits is 20 psi to 80 psi (0.14 MPa to 0.55 MPa), but the pressure can range from 300 psi to 3000 psi (2.07 MPa to 20.7 MPa) while bruxing. This, in turn, places significantly more stress on the muscles of mastication; and, as they are overworked, MPS and the formation of trigger points ensue.
Masseter Hypertrophy
When examining a patient for TMD-related MPS caused by bruxism, a clinician may often find trigger points in the masseter muscles. Patients frequently present with such severe hypertrophy of the masseter muscles that the bulge in the muscle causes facial distortion. Masseter hypertrophy can be treated with BoNT-A injections using the same protocol used to treat TMD pain in the masseter. The injections will decrease the intensity of the contractions, and as the muscle begins to relax, the patient will not be able to clench with the same force. In addition to pain reduction, the end result is a desirable slenderizing of the face as the masseter loses its hypertrophic appearance (Figure 5 and Figure 6).19
Obstructive Sleep Apnea
Obstructive sleep apnea (OSA) occurs when repeated episodes of complete or partial blockage of the upper airway happen during sleep. During an OSA episode, the diaphragm and chest muscles work harder to open the obstructed airway and pull air into the lungs. A patient with OSA is likely to have TMD and nocturnal bruxism.20-22
The American Academy of Dental Sleep Medicine classifies sleep bruxism as a sleep-related movement disorder.23 A home bruxism and sleep study monitor is a cost-effective way for a dentist to obtain data on the patient’s sleep apnea and diagnose bruxism. The information that can be collected from this test includes oxygen levels, masseter muscle activity for bruxism, pulse, airflow, snoring, chest movement, and body position during sleep.
Once the data obtained from a bruxism/sleep study confirms that the apnea-hypopnea index (AHI) may indicate the presence of OSA, the dental practitioner should contact the patient’s physician, who can make the definitive medical diagnosis of OSA. The practitioner can then intervene. For patients with mild or moderate OSA, dental appliances or oral mandibular advancement devices that prevent the tongue from blocking the throat and/or advance the lower jaw forward can be made. These devices help keep the airway open during sleep. In many cases, the patient will no longer have nocturnal bruxism once treated.24
Headaches
The association between sleep, bruxism, TMD, and headaches has long been recognized.25 Headaches afflict a large portion of the population and, with varying severity, can result in discomfort, disruption of daily activity, lost days at work, and, occasionally, debilitating pain. Although about 30% of headache sufferers are periodically functionally impaired, many do not seek medical care.26,27 Typically, patients report various headache symptoms in conjunction with a dental examination.
Tension-Type Headaches and Migraine
The tension-type headache (TTH) is the most common primary head pain, and most of the population will experience this at least once in their lifetimes.28 Findings from the dental examination will generally reveal that pain generating from the masticatory musculature can be episodic and chronic and may be indistinguishable clinically and therapeutically from migraine. It is likely that some TTHs and, correspondingly, some TMDs represent a variant form of migraine or have a migraine-like component.29,30 In fact, there is a somewhat overlapping diagnosis of headache attributed to TMD in accordance with the diagnostic/TMD criteria and the International Headache Society criteria.31,32
The relationship between a TMD and headache is well recognized in the literature. Patients receiving a diagnosis of either migraine or TTHs, which may be caused by myalgia of the temporalis muscle, will have signs and symptoms consistent with TMD. Strengthening this relationship between TMD and headache is the fact that patients who have undergone treatment for TMD report a decrease in symptoms of headache. Recent evidence suggests that patients who have a diagnosis of vascular or migraine headache have a higher prevalence of TMD as a contributing cause of their pain than the general population. In addition to the trigeminal nerve, the facial nerve and muscles of facial expression are intricately involved with the headache/TMD continuum.33-35
On October 15, 2010, the FDA approved BoNT-A injections to prevent headaches in adults with chronic migraine. The treatment protocol involves selective relaxation of hyperfunctional muscle of mastication and facial muscles with BoNT-A. The idea is to administer the smallest, effective dose necessary to relieve the pain; the dosage is based on each patient’s response to the therapy. Again, dentists who are considering administering BoNT-A injections are urged to take an appropriate hands-on training course and follow the rules of the state where they practice.
The mechanism with which BoNT-A relieves migraine pain is not clearly understood. It is thought that because it controls unconscious jaw movement, it lessens the load on the muscles and, thus, alleviates grinding-related headaches.36 However, the release of neuropeptides, particularly calcitonin gene-related peptide (CGRP), is considered an integral component in the pathophysiology of migraine.37 In addition to its effect on the autonomic nervous system, it has been shown that BoNT-A can directly decrease the amount of CGRP released from trigeminal neurons. This finding suggests BoNT-A may also reduce headache pain because it has a direct effect on the central nervous system.37
Cervicogenic Headaches
Neck pain and cervical muscle tenderness are common symptoms of primary headache disorders. A diagnosis of cervicogenic headaches (CGHs) is made when head pain arises from bony structures or soft tissues of the neck. This can be a perplexing pain disorder that is refractory to treatment if it is not recognized. The condition’s pathophysiology is likely referred from one or more muscular, neurogenic, osseous, articular, or vascular structures in the neck. It is often a sequela of head or neck injury but may also occur in the absence of trauma. The clinical features of CGH may mimic those commonly associated with primary headache disorders such as TTH or migraine, and, as a result, distinguishing among these headache types can be difficult.38
The diagnosis of CGH can often be made after a careful history and physical examination is performed. The criteria may include one or more of the following symptoms: moderate or severe pain reported in the occipital, frontal, temporal, orbital, neck, and back regions; intermittent or chronic pain generally deep and non-throbbing; head pain triggered by neck movements; or restricted range of motion in the neck. Patients with CGH will usually present with a forward head posture. Muscular trigger points are usually found in the suboccipital, cervical, and shoulder musculature. These trigger points can also refer pain to the head when manually or physically stimulated.38,39
Studies indicate 44.1% of patients with CGH have MPS-related TMD. In addition, it has been shown that patients with CGH who receive TMD therapy had increased range of motion in the neck. On palpation, trigger points are usually found in the suboccipital, cervical, and shoulder musculature. When manipulated, these areas often refer pain to the head, even though the neck musculature is the source of the pain.39 Like other MPS-related pain, this area responds well to BoNT-A injections.
Conclusion
TMD is a collection of clinical entities that are often painful and disabling. Yet, they are self-limiting and usually respond to conservative therapy such as injection with BoNT-A. Basic principles of management to reduce pain and restore range of motion will reduce disability and often contribute to reducing primary headache disorder if it coexists.
In addition to controlling TMD, serious dental problems such as destruction of the teeth or restorations, tooth mobility, and periodontal disease, all caused or exacerbated by bruxism, can be avoided. Other benefits of TMD treatment include elimination of nocturnal bruxism, reduction in jaw tension, and decreased chronic neck and shoulder pain. Dentists who suspect a TMJ or bruxism condition should have the patient tested with a home bruxism/sleep monitor test before performing any treatment to obtain a baseline reading of the patient’s bruxism episodes and AHI.
Patients with chronic orofacial pain will often seek the help of their dentists when symptoms arise. Didactic and hands-on education is recommended to become proficient in the treatment of TMD and orofacial pain in everyday dental practice.
About the Authors
Lisa Germain, DDS, MScD
Faculty
American Academy of Facial Esthetics
Private Practice
New Orleans, Louisiana
Louis Malcmacher, DDS, MAGD
President
American Academy of Facial Esthetics
Private Practice
Bay Village, Ohio
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
Disclosures
Dr. Germain had no disclosures to report. Dr. Malcmacher is President of the American Academy of Facial Esthetics and a consultant for STATDDS™.
References
1. Balasubramaniam R, Klasser GD. Orofacial pain syndromes: evaluation and management. Med Clin North Am. 2014;1998(6):1385-1405.
2. Mersky H, Bogduk N. Classification of Chronic Pain. 2nd ed. Seattle, WA: IASP Press; 1994:59-71.
3. Okeson JP. Bell’s Orofacial Pains. 6th ed. Chicago, IL: Quintessence Publishing Co, Inc.; 2005:162-167.
4. Lipton J, Ahip J, Larach-Robonson D. Estimated prevalence and distribution of orofacial pain in the United States. J Am Dent Assoc. 1991; 124(10):115-121.
5. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, and temporomandibular disorder. Arch Intern Med. 2000;160(2):221-227.
6. Okeson JP. Management of Temporomandibular Disorders and Occlusion. 5th ed. St Louis, MO: Mosby; 2003:10-79.
7. Turp J, Schindler H. The dental occlusion as a suspected cause for TMD’s: Epidemiological and etiological considerations. J Oral Rehabil. 2012;39(7):502-512.
8. Luther F. TMD and occlusion. TMD and occlusion part II. Damned if we don’t? Functional occlusal problems: TMD epidemiology in a wider context. Br Dent J. 2007;202(1):E3.
9. Turp JC, Kowalski CJ, Stohler CS. Temporomandibular disorders—pain outside the head and face is rarely acknowledged in the chief complaint. J Prosthet Dent. 1997;78(6):592-595.
10. Klasser GD, Bassiur J: Differences in reported medical conditions between myogenous and arthrogenous TMD patients and its relevance to the general practitioner. Quintessence Int. 2014;45(2):157-167.
11. Schiffman E, Ohrbach R, Truelove E, et al; for the International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28(1)2014:6-27.
12. Simons DG, Travell JG, Simons LS, Cummings BD. Myofascial Pain and Dysfunction: The Trigger Point Manuel, Vol 1 - The Upper Half of the Body. Baltimore, MD: Williams & Wilkins; 1999:11-178.
13. Syrop S. Initial management of temporomandibular disorders. Dent Today. 2002;21(8):52-57.
14. Stohler CS, Zarb GA. On the management of temporomandibular disorders: a plea for a low-tech, high-prudence therapeutic approach. J Orofac Pain. 1999;13(4):255-261.
15. Greene CS, Laskin DM. Long term evaluation for myofascial pain dysfunction syndrome: a comparative analysis. J Am Dent Assoc. 1983:107 (2):235-238.
16. Management of temporomandibular disorders. National Institutes of Health Technology Assessment Statement. 1966;127(11):1595-1606.
17. Ludlow CL, Hallett M, Rhew K, et al. Therapeutic use of type F botulinum toxin. N Engl J Med. 1992;326:349-350.
18. Malcmacher L. Botox therapy for every dental practice. Dent Today. 2009;28(8):101-103.
19. Malcmacher L. Botulinum toxin for frontline TMJ syndrome and dental therapeutic treatment. Dental Economics. 2013:93-99.
20. Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109 (6):e26-e50.
21. Glaros AG. Incidence of diurnal and nocturnal bruxism. J Prosthet Dent. 1981;45(5):545-549.
22. Goulet JP, Lund JP, Montplaisir J, et al. Daily clenching, nocturnal bruxism, and stress and their association with TMD symptoms. J Orofac Pain. 1993;7:89.
23. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL: The American Academy of Sleep Medicine, 2014.
24. Simmons JH, Prehjn R. Airway protection: The missing link between nocturnal bruxism and obstructive sleep apnea. Sleep. 2009;32(abstract suppl):A218.
25. Sharav Y, Benoliel R, eds. Orofacial Pain and Headache. 2nd ed. Hanover Park, IL: Quintessence Pub; 2015:123-165.
26. Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormone, sleep pattern and relation to lifestyle. Pain. 1993;53(1):65-72.
27. Saper JR, ed. Clinician’s Manual on Headache. Philadelphia, PA: Science Press; 1995:1-86.
28. Schiffman E, Halet D, Baker C, Lindgren B. Diagnostic criteria for screening headache patients for temporomandibular disorders. Headache 1995;35(3):121-135.
29. Schellhas KP, Wilkes CH, Baker CC. Facial pain, headache, and temporomandibular joint inflammation. Headache 1989; 29(4):228-231.
30. Haley D, Schiffman E, Baker C, Belgrade M. The comparison of patients suffering from temporomandibular disorders and a general headache population. Headache 1993;33(4):210-213.
31. Schokker RP, Hansson TL, Ansik BJ. Differences in headache patients regarding response to treatment of the masticatory system. J Craniomandib Disord. 1990;4(4):228-232.
32. Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons’ integrated hypothesis of trigger point formation. Curr Pain Headache Rep. 2004;8(6):468-475.
33. Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders(DC/TMD) for clinical and research applications: recommendation of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28(1):6-27.
34. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013:33(9):629-808.
35. Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8(2):136-142.
36. Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2005;43(suppl 1):9-15.
37. Edvinsson L. Calcitonin gene-related peptide (CGRP) and the pathophysiology of headache: therapeutic implications. CNS Drugs. 2001;15(10):745-753.
38. Haldeman S, Dagenais S. Cervicogenic headaches: a critical review. Spine J. 2001;1(1):31-46.
39. von Piekartz H, Lüdtke K. Effect of treatment of temporomandibular disorders (TMD) in patients with cervicogenic headache: a single-blind, randomized controlled study. Cranio. 2011;29(1):43-56.